
How affective feelings evolved in human and animal brains remains one of the central scientific mysteries of our field. To illuminate such deeply psychological question, we have few strategic options but to seek relevant neuroscientific evidence from other animals. There remain barriers to this. It is still commonly believed that “We will never know what an animal feels” (LeDoux 2012, 666), a bias that closely aligns with classical behaviorist and ethological traditions (e.g., consider Nobel Laureate Nico Tinbergen’s assertion in his Study of Instinct (1951, 4) that “[b]ecause subjective phenomena cannot be observed objectively in animals, it is idle to claim or deny their existence.”).
Such skepticism was scientifically “reasonable” before the advent of modern neuroscience, but continuing skepticism in the current era overlooks abundant affective neuroscience data for animal emotional feelings—namely, that animals find artificial activation of what I call primary-process subcortical emotional systems to be rewarding and punishing. Clearly, these effects do not arise from neocortical read-out processes such as working memory, but rather directly from deep subcortical networks which generate instinctual emotional behaviors (e.g., self-stimulation of the SEEKING system survives radical neo-decortication at birth—Huston & Borbély, 1973). Thus, the affective neuroscience perspective is that animal research provides abundant evidence for the subcortical sources of emotional feelings in all mammals (Panksepp, 1982, 1985, 1998).
This argument has been laid out simplest in Panksepp (2011). We can evoke at least 7 emotional patterns with subcortical Deep Brain Stimulation (DBS), each associated with distinct forms of arousal that are either rewarding (SEEKING, LUST, CARE and PLAY, all of which are evoked along the trajectory of the Medial Forebrain Bundle (MFB)) or punishing (RAGE, FEAR, and PANIC) (see Panksepp & Biven, 2012 for a recent review).
It is important to note that the capitalizations are meant to highlight that what is being referred to are primary-process affective systems of the brain, which are next to impossible to study incisively in humans. Indeed, to sustain conceptual clarity, I divide the evolved brain mechanisms critical for understanding affective phenomena into a tripartite level of analysis—primary (raw instinctual-affective), secondary (unconscious learning and memory related processing) and tertiary (higher cognitive manifestations) levels.
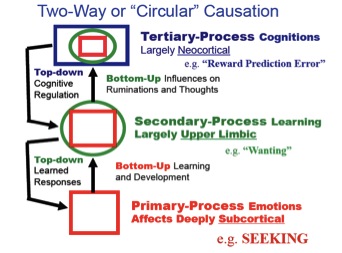
Nested hierarchies of control within the brain. Primary-processes are depicted as squares (red; e.g. SEEKING), secondary processes are depicted as circles (green; e.g. Kent Berridge’s wanting), and tertiary processes are depicted as rectangles (blue; Wolfram Schultz’s “reward prediction error”). The color-coding aims to convey the manner in which lower brain functions are integrated into higher brain functions to eventually exert top-down regulatory control. The figure shows the bottom-up and top-down (circular) causation that is proposed to operate in every primal emotional system of the brain
As highlighted in Figure 1, each of these levels needs distinct nomenclatures for clear discourse to emerge, which remains especially difficult in areas such as emotion research where few scientifically agreed upon definitions exist. Abundant evidence for SEEKING, RAGE, FEAR, LUST, CARE, PANIC and PLAY systems are detailed elsewhere (Panksepp, 1998). Human PET-based brain imaging (more appropriate for envisioning affective states than fMRI) has seen such systems in human brains (see Figure 2, based on work by Damasio, et al., 2000).
Work on these, and other affective systems (e.g. sensory and homeostatic), should help us understand how “reinforcements” are engendered in the brain, promoting learning and memory. The resulting secondary-process levels of behavioral complexities, especially well detailed in studies of fear conditioning (LeDoux, 2012) may arise from neural “Laws of Affect” whereby fluctuating primary-process affective feeling circuits control learning and solidification of memories—as in the transformation of “silent-synapses” in dynamic changes in glutamatergic transmission (see Chapter 6 Panksepp & Biven, 2012). Claims that primary-process emotional arousals are not experienced in animals need to be cashed out with demonstrations that rewards and punishments can work effectively in humans without any associated experienced affective changes.
![Figure 2 Overview of brain arousals and inhibitions. An overview of brain arousals (reds and yellows) and inhibitions (purples) depicted on lateral surfaces of the right and left hemispheres (top of each panel) and medial surfaces of the corresponding hemispheres (bottom of each panel), while humans experience various basic emotions evoked by autobiographical reminiscing. Upper left: sadness/GRIEF; upper right: happiness/JOY; lower left: anger/RAGE; lower right: anxiety/FEAR (data from Damasio, et al. [38]; overall patterns of activation and inhibition (this figure graciously provided by Antonio Damasio). To highlight the directionality of changes, as monitored by changes in blood flow, inhibitions are indicated by downward arrows (predominating in neocortical regions), while arousals are depicted by upward arrows (predominantly in subcortical regions where emotional behaviors can be evoked by brain stimulation in animals)](http://emotionresearcher.com/wp-content/uploads/2013/11/four-emotions.jpg)
An overview of brain arousals (reds and yellows) and inhibitions (purples) depicted on lateral surfaces of the right and left hemispheres (top of each panel) and medial surfaces of the corresponding hemispheres (bottom of each panel), while humans experience various basic emotions evoked by autobiographical reminiscing. Upper left: sadness/GRIEF; upper right: happiness/JOY; lower left: anger/RAGE; lower right: anxiety/FEAR (data from Damasio, et al. 2000; overall patterns of activation and inhibition (this figure graciously provided by Antonio Damasio). To highlight the directionality of changes, as monitored by changes in blood flow, inhibitions are indicated by downward arrows (predominating in neocortical regions), while arousals are depicted by upward arrows (predominantly in subcortical regions where emotional behaviors can be evoked by brain stimulation in animals)
My own work has explicitly sought to clarify cross-species, primary-process emotional systems and the feelings they generate. The critical fact that has permitted this is our ability to evoke coherent emotional response patterns with Deep Brain Stimulation. The affective evaluation of those evoked states is achieved with traditional operant learning procedures (conditioned approach and escape), which can at the very least tell us whether the feelings are positive or negative, with the possibility of discriminating different rewarding feelings (Stutz, et al., 1974) and relating such data to human affective experiences (Panksepp, 1985).
In my estimation, the continuing neglect, indeed denial, of affective processes in animal brain research has prevented us from envisioning how the mind was constructed in brain evolution, where subcortical functions are foundational for all the rest (Solms & Panksepp, 2013). It also explains the failure of animal research to yield new psychiatric medicines, all of which, since the initial breakthrough starting 60 years ago, have been discovered by chance. The subsequent widespread use of animal behavioral models of psychiatric disorders has yet to yield any new psychiatric medicines. I predict we can do better when we begin to scientifically understand our own primal emotional feelings through cross-species research.
Indeed, that was my main reason for investing in primary-process affective neuroscience strategies. Based on this understanding, we are currently evaluating three new interventions for human depression: i) the discovery of new antidepressants that can facilitate social-joy as studied through ancestral PLAY processes of the brain (Burgdorf, et al., 2011), ii) the treatment of depression by stimulating brain SEEKING (“enthusiasm” in the vernacular) urges, through deep brain stimulation (DBS) of the human medial forebrain bundle (MFB) (Coenen, et al., 2012), and iii) the use of safe opioids such as buprenorphine for anti-depressant and anti-suicidal effects, by reducing psychological pain arising from brain PANIC arousal (Yovell, Panksepp, et al., in progress). With an understanding of the opioid neurochemistries of separation-distress and social-bonding, some progress has also been made in treating autism (Bouvard, et al., 1995), and through the study of PLAY, new psychosocial treatments for ADHD are being envisioned (Panksepp, 2007)
Affective neuroscience also offers a vision of how consciousness evolved: At the beginning there emerged raw affects, whose function was to anticipate survival issues: All positive affects inform organisms, unconditionally, that they are proceeding on paths of survival. All negative feelings inform organisms, also unconditionally, about probable paths of destruction. These affective “intuitions” are cashed out—extended in time–through learning and memory, becoming mental appraisals as they mix with abundant tertiary-process higher cortical processes, which emerge via culturally guided developmental learning and epigenetic processes. This vision can diminish disagreements among people working on different levels of analysis of psychological processes of common interest.
A cross-species affective neuroscience allows us to integrate findings from basic animal brain research and constructivist views of the human mind, by recognizing how investigators are working on common interests at different levels of brain-mind organization. That these views are often at odds reflects a failure of our educational enterprises to integrate scientifically meaningful images of bottom-up developmental processes with maturation of top-down, thought-laden regulatory processes. One of the finest, and least appreciated, pieces of good news is that the neocortex at birth resembles a tabula rasa more than a conglomerate of evolutionarily specialized modules. All neocortical specializations, even our capacity for vision, arise through early sensory experiences and epigenetic moldings of higher brain functions. Constructivism works best in our understanding of higher mental functions, and hence what makes humans unique; evolutionary perspectives work best in understanding the subcortical specializations that all mammals share. Such disparate views can be integrated (see Zachar & Ellis, 2012 for relevant discussions).
It may be wise for emotion-science to wholeheartedly welcome the good news: We can finally comprehend the general neural principles that undergird our emotional affects by studying homologous processes in other animals. This knowledge has allowed us to develop new biological ways to understand and treat psychiatric disorders. One of our lead antidepressant molecules, GLYX-13, discovered by taking the social-joy (PLAY arousal) of other animals seriously, in the form of “rat laughter” (Burgdorf, et al., 2011), is currently in FDA approved Phase 2b human testing, with promising results from the Phase 2a “proof of concept” studies already completed: http://www.drugs.com/clinical_trials/naurex-s-novel-antidepressant-glyx-13-recognized-one-windhover-s-top-10-neuroscience-projects-watch-10010.html. If that mind medicine, which may facilitate the progression of psychotherapy, by facilitating learning (i.e., GLYX-13 facilitates neuronal long-term potentiation, an electrophysiological marker of learning), ever comes to market, it may be the first time neuroscientific research into brain emotional processes, as opposed to mere serendipity, has yielded an effective way to treat any human psychiatric disorder. This was facilitated by the first validated psychoassay for positive social affect—namely systematic tickling of rats to generate an ancestral form of laughter.
For a general introduction to Jaak’s life and career, take a look at the following two recent interviews:
- Discover Magazine: The Man Who Makes Rats Laugh: Jaak Panksepp
- Washington State Magazine: The Animal Mind Reader
You can also check out Jaak’s foreword to his wife’s Anesa Miller’s new book, entitled To Boldy Go.
References
Bouvard, M.P., Leboyer, M., launay, J.-M., Recasens, C., Plumet, M.-H., Waller-Perotte, D., Tabuteau, F., Bondoux, D., Dugas, M., Lensing, P., and Panksepp, J. (1995) Low-dose naltrexone effects on plasma chemistries and clinical symptoms in autism: A double-blind, placebo-controlled study. Psychiatry Research, 58, 191-201
Burgdorf, J., Panksepp, J., & Moskal, J. R. (2011). Frequency-modulated 50kHz ultrasonic vocalizations: a tool for uncovering the molecular substrates of positive affect. Neuroscience & Biobehavioral Reviews, 35(9), 1831-1836.
Coenen, V.A., Panksepp, J., Hurwitz, T.A., Urbach, H., & Mädler, B. (2012) Human medial forebrain bundle (MFB) and anterior thalamic radiation (ATR): Diffusion tensor imaging of two major subcortical pathways that may promote a dynamic balance of opposite affects relevant for understanding depression. The Journal of Neuropsychiatry and Clinical Neurosciences. 24, 223-236.
Davis, K. L. & Panksepp, J. (2011). The brain’s emotional foundations of human personality and the Affective Neuroscience Personality Scales. Neuroscience & Biobehavioral Reviews, 35(9), 1946-1958.
Huston, J.P. & Borbély, A.A. (1973). Operant conditioning in forebrain ablated rats by use of rewarding hypothalamic stimulation. Brain Research, 50, 467-72.
LeDoux, J. (2012). Rethinking the emotional brain. Neuron, 73(4), 653-676.
Panksepp, J. (1982) Toward a general psychobiological theory of emotions. The Behavioral and Brain Sciences, 5, 407-467.
Panksepp, J., (1985) Mood changes: In P.J. Vinken, G.W. Bruyn, & H.L. Klawans (Eds). Handbook of Clinical Neurology (Revised Series). Vol. 1. (45): Clinical Neuropsychology. Amsterdam: Elsevier Science Publishers, pp. 271-285.
Panksepp, J. (1998). Affective Neuroscience: The Foundations of Human and Animal Emotions. New York: Oxford University Press.
Panksepp, J. (2005). Affective consciousness: Core emotional feelings in animals and humans. Consciousness & Cognition, 14, 19-69.
Panksepp, J., (2007). Can PLAY diminish ADHD and facilitate the construction of the social brain. Journal of the Canadian Academy of Child and Adolescent Psychiatry, 10: 57-66.
Panksepp, J. (2011). Cross-species affective neuroscience decoding of the primal affective experiences of humans and related animals. PLoS One, 6(8): e21236.
Panksepp, J. & Biven, L. (2012). Archaeology of Mind: The Neuroevolutionary Origins of Human Emotions. New York: Norton.
Solms, M. & Panksepp, J. (2012). The “Id” knows more than the “Ego” admits: Neuropsychoanalytic and primal consciousness perspectives on the interface between affective and cognitive neuroscience. Brain Science, 2, 147-175.
Stutz, R.M., Rossi, R.R., Hastings, L., & Brunner, R.L. (1974). Discriminability of intracranial stimuli: The role of anatomical connectedness. Physiology & Behavior, 12, 69-73.
Zachar, P., & Ellis, R. (Eds.). (2012). Categorical versus dimensional models of affect: A seminar on the theories of Panksepp and Russell (Consciousness and Emotion book series). Amsterdam, the Netherlands: John Benjamins





