Luiz Pessoa, Department of Psychology, University of Maryland
 For over a century, neuroscience has held tight to a framework of computations as performed by brain regions. Accordingly, when brain scientists sought to understand the brain basis of emotion, a search for key areas was initiated. With time, several of them were put forward, including emotion “centers” such as the hypothalamus and amygdala. The continued search for the “emotional brain” eventually led to an expanded list of emotion-related subcortical and cortical areas among which the orbitofrontal cortex, the anterior insula, the anterior cingulate cortex, in addition to the ones above, are very popular ones. Depending on how one counts, the list can easily add up to more than a dozen regions.
For over a century, neuroscience has held tight to a framework of computations as performed by brain regions. Accordingly, when brain scientists sought to understand the brain basis of emotion, a search for key areas was initiated. With time, several of them were put forward, including emotion “centers” such as the hypothalamus and amygdala. The continued search for the “emotional brain” eventually led to an expanded list of emotion-related subcortical and cortical areas among which the orbitofrontal cortex, the anterior insula, the anterior cingulate cortex, in addition to the ones above, are very popular ones. Depending on how one counts, the list can easily add up to more than a dozen regions.
Two issues are immediately evident. The first is that the list is extremely difficult to define. Consider, for instance, the problem of defining the “limbic” brain, an effort that has essentially failed, although the term continues to be as popular as ever – unfortunately. The second, and the one I would like to discuss here, is that recent understanding of anatomical pathways reveals architectural features that show that cortex and subcortex are part of a connectivity system that allows for massive distribution and aggregation of neural signals (Swanson, 2000; Modha & Singh, 2010). It is thus not surprising that much research has revealed that brain regions are involved in many functions, and that similar functions are performed by many regions. The mapping between structure and function is thus both pluripotent (one-to-many) and degenerate (many-to-one) (Edelman & Gally, 2001).
Based on these notions, a network perspective is needed for the understanding of the interactions between emotion, motivation, perception, and cognition (Grossberg, 1980; Barbas, 1995; Damasio, 1999; Mesulam, 1999; Pessoa, 2008; 2013). Briefly, networks of brain regions collectively support behaviors: the network itself is the unit, not the brain region. Processes that support behavior are not implemented by an individual area, but rather by the interaction of multiple areas, which are dynamically recruited into multi-region assemblies (Figure 1).
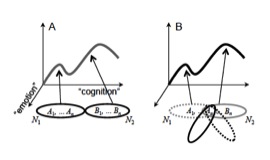
Conceptual proposal for the relationship between anatomical regions, networks, and cognitive-emotional” behaviors. (A) Brain areas (for example, A1 and B1) are grouped into networks (ellipses). At a given time, a network will support a specific cognitive-emotional behavior (position along the curve). (B) A given brain area will participate in multiple networks (at different times). Thus, it will contribute to different cognitive-emotional processes depending on the regions it is partnering with
However, importantly, whereas a network perspective is needed for a fuller characterization of the mind–brain, it should not be viewed as a panacea. For one, the challenges posed by the many-to-many mapping between regions and functions is not dissolved by the network perspective. Indeed, one should not anticipate a one-to-one mapping when the network approach is adopted – counter to the recent trend of labeling networks with specific functions. Additionally, decomposition of brain regions in terms of meaningful clusters, such as the ones generated by recent “network science” algorithms (Newman, 2010), does not by itself reveal “true” subnetworks. Given the complex and multi-relational relationship among regions, multiple decompositions will offer different viewpoints of how to understand their interdependency.
Within a distributed computation perspective, the emphasis shifts from attempting to understand the brain one region at a time, to understanding how coalitions of regions support the mind–brain. Insofar as brain regions are not the unit of interest, they should not be viewed as “cognitive” or “emotional”. Traditionally, however, regions whose function involves homeostatic processes and/or bodily representations have been frequently viewed as “emotional”, whereas regions operating on more abstract information – such as those involved in problem solving and planning – have been viewed as “cognitive”.
Consider the extensive communication between the amygdala and visual cortex (incidentally, an architectural feature seen in primates only): efferent amygdala projections reach nearly all levels of the visual cortex (Amaral et al., 2003). Thus, visual processing takes place within a context that is defined by signals occurring in the amygdala (as well as the orbitofrontal cortex, pulvinar, and other regions), including those linked to affective significance (Pessoa & Adolphs, 2010). Consider also the connectivity of prefrontal cortex. Although the amygdala is not connected to all PFC territories, a “one-step” property of amygdala–prefrontal connectivity is present: amygdala signals reach nearly all prefrontal regions with a single additional connection within PFC (e.g., pathways between medial and lateral PFC; see Averbeck & Seo, 2008). Thus, cognitive–emotional interactions abound in the prefrontal cortex.
More generally, given inter-region interactivity, and the fact that networks intermingle signals of diverse origin, although a characterization of brain function in terms of networks is needed, the networks themselves are best conceptualized as neither “cognitive” nor “emotional”. The preceding discussion anticipates an important notion: emphasizing interactions among brain regions that are supported by direct, strong structural connections is misleading. Understanding structural connectivity is essential, but it is not sufficient. Although, at first glance, the notion of an architecture anchored in physical connections is clear cut, the boundary between anatomy and function quickly blurs when we consider specific anatomical factors such as the receptor subtypes involved, the presence and proportion of excitatory and inhibitory interneurons, and the strength of the connections. The existence of complex circuits with multiple feedforward and feedback connections and the existence of diffuse projection systems further complicates the picture.
Thus, to understand how regions and networks contribute to brain function, it is necessary to identify the way regions are functionally connected. Devised to characterize how neurons interact, functional connectivity was initially defined as the “temporal coherence” among the activity of different neurons, as measured by cross-correlating their spike trains (Gerstein & Perkel 1969); or, more generally, the “temporal correlation between neurophysiological (functional) measurements made in different brain areas” (Friston et al., 1993). Understanding functional connectivity is vital, because it will frequently deviate from that expected from simply considering structural information. Overall, architectural features guarantee the rapid integration and distribution of information even when robust structural connections are not present, and support functional interactions that are heavily context dependent.
What are the implications of these “network ideas” for understanding emotion? Together, they suggest that the mind–brain is not decomposable in terms of categories such as “emotion” and “cognition”. Although versions of this idea have been advanced by others too (e.g., Damasio, 1999; Mesulam, 1999; Lindquist et al., 2010), the present proposal differs from these in important ways as  outlined in a recent book entitled The Cognitive-Emotional Brain: From Interactions to Integration (Pessoa, 2013). In a nutshell, the neural basis of emotion and cognition should be viewed as governed less by properties that are intrinsic to specific sites and more by contextually determined interactions among multiple brain regions. In this sense, emotion and cognition are functionally integrated systems, namely, they more or less continuously impact each other’s operations (see Bechtel & Richardson 2010). What ensue are organisms that navigate their ecological niches successfully.
outlined in a recent book entitled The Cognitive-Emotional Brain: From Interactions to Integration (Pessoa, 2013). In a nutshell, the neural basis of emotion and cognition should be viewed as governed less by properties that are intrinsic to specific sites and more by contextually determined interactions among multiple brain regions. In this sense, emotion and cognition are functionally integrated systems, namely, they more or less continuously impact each other’s operations (see Bechtel & Richardson 2010). What ensue are organisms that navigate their ecological niches successfully.
References
Amaral, D. G., Behniea, H., and Kelly, J. L. (2003). Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience, 118(4), 1099–1120.
Averbeck, B. B., and Seo, M. (2008). The statistical neuroanatomy of frontal networks in the macaque. PLoS Computational Biology, 4(4), e1000050.
Barbas, H. (1995). Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neuroscience and Biobehavioral Reviews, 19(3), 449–510.
Bechtel, W., and Richardson, R. C. (2010). Discovering Complexity: Decomposition and Localization as Strategies in Scientific Research (2nd ed.). Cambridge, MA: MIT Press.
Damasio, A. R. (1999). The Feeling of What Happens: Body and Emotion in the Making of Consciousness. New York: Harcourt Brace.
Edelman, G. M., and Gally, J. A. (2001). Degeneracy and complexity in biological systems. Proceedings of the National Academy of Sciences of the United States of America, 98(24), 13763–13768.
Friston, K. J., Frith, C. D., Liddle, P. F., and Frackowiak, R. S. (1993). Functional connectivity: The principal-component analysis of large (PET) data sets. Journal of Cerebral Blood Flow and Metabolism, 13(1), 5–14.
Gerstein, G. L., and Perkel, D. H. (1969). Simultaneously recorded trains of action potentials: Analysis and functional interpretation. Science, 164(3881), 828–830.
Grossberg, S. (1980). How does a brain build a cognitive code? Psychological Review, 87(1), 1–51.
Lindquist, K.A., Wager, T.D., Kober, H., Bliss-Moreau, E., Barrett, L.F. (2012). The brain basis of emotion: a meta-analytic review. Behavioral & Brain Sciences, 35(3), 121-143.
Mesulam, M.-M. (1981). A cortical network for directed attention and unilateral neglect. Annals of Neurology, 10, 309–325.
Modha, D. S., and Singh, R. (2010). Network architecture of the long-distance pathways in the macaque brain. Proceedings of the National Academy of Sciences of the United States of America, 107(30), 13485–13490.
Pessoa, L. (2008). On the relationship between emotion and cognition. Nature Reviews Neuroscience, 9(2), 148–158.
Pessoa, L. (2013). The cognitive-emotional brain: From interactions to integration. Cambridge: MIT Press.
Pessoa, L., and Adolphs, R. (2010). Emotion processing and the amygdala: From a “low road” to “many roads” of evaluating biological significance. Nature Reviews. Neuroscience, 11(11), 773–783.
Swanson, L. W. (2000). Cerebral hemisphere regulation of motivated behavior. Brain Research, 886(1–2), 113–164.




